What Rights Do Nursing Home Residents Have?
Who Is Eligible to File a Camp Lejeune Claim?
Camp Lejeune Claims Portal Now Available
Recent Articles
What Documents Are Required to File a Camp Lejeune Claim?
For more than 30 years, the water at Camp Lejeune in North Carolina contained toxic chemicals. Servicemembers, their family members, and civilians unknowingly drank and bathed in contaminated water for decades before the military eventually shut down the affected wells. Historically, veterans couldn’t file Camp…
California Women’s Prison Plagued with Sex Abuse Has Closed
The Federal Correctional Institution in Dublin, California (FCI-Dublin) served as the sole women-only federal prison east of the Rocky Mountains for decades, and its list of former residents includes Heidi Fleiss, Patty Hearst, Squeaky Fromme, and Felicity Huffman. However, in recent years, FCI-Dublin has been…
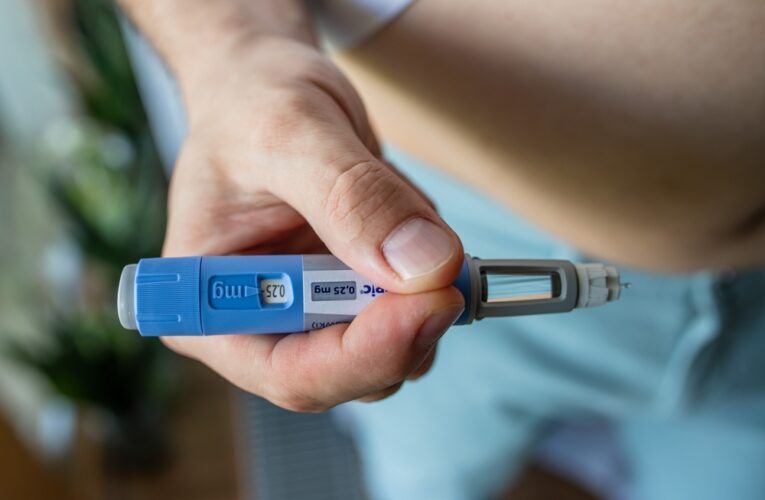
Do I Need a Lawyer for Diabetes Weight Loss Drug Injuries?
For millions of Americans diagnosed with diabetes, this may mean a lifetime of uncertainty, monitoring their diet and blood sugar levels. Some may require medication to treat their diabetes when lifestyle changes are not effective. For those who need medical assistance to control their blood…
Settlement Reached in CPAP Personal Injury Lawsuits
Philips Respironics will pay $1.1 billion to people who used the company’s CPAP, BiPAP, and ventilator devices and suffered injuries from foam degradation. The settlement, announced earlier this week, will resolve thousands of CPAP lawsuits from people injured after using Philips DreamStation machines. Philips did…
Who Is Eligible to File a Camp Lejeune Claim?
The deadline for filing a claim under the Camp Lejeune Justice Act (CLJA) is fast approaching. Almost two years ago, President Joe Biden signed the CLJA into law, and the legislation opened a legal window for victims of Camp Lejeune’s contaminated water to receive financial…
$100 Million Settlement with DOJ over Larry Nassar Cases
As the country’s top gymnasts are feeling the pressure to qualify for the Summer Olympics in Paris, the story of how Larry Nassar, the former USA Gymnastics doctor who sexually abused hundreds of young women under the guise of medical treatment, is once again in…
How a Lawyer Can Help with CPAP Injuries
For the 25 million Americans who experience sleep apnea, using a CPAP machine at night can help treat this sleep disorder. Sleep apnea can cause people to have irregular and inconsistent breathing while sleeping, while some start and stop breathing throughout the night. The constant…
$60 Million Verdict Could Lead to More NEC Baby Formula Lawsuits
An Illinois jury awarded $60 million last month to a woman whose premature baby died from necrotizing enterocolitis (NEC) after consuming baby formula. The jury found that Mead Johnson, the manufacturer of the popular formula brand Enfamil, acted negligently and failed to warn consumers about…